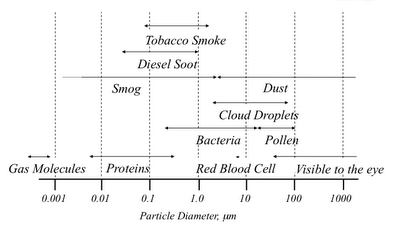
Aerosol particles cover a wide range in size from a small cluster of molecules to a piece of playa dust. I use the simple diagram below to show how aerosols fit into the size of things. The bottom axis shows particle diameter in micrometers (0.0000001 meters), and ranges from 0.001 microns (or 1 nm) to 1000 microns. Individual particles become visible to the eye at around 30 microns I believe.
Note that smog and dust are shown as two different types of particles. This is partly due to the difference between photochemical smog and existing dust that is not cooked up in the atmosphere. But there is another important reason. The particulate air pollution in smog is formed from gas molecules that react in different ways to condense and form the particle. The particles in the dust size range are formed by mechanical processes that turn larger material into smaller particles.
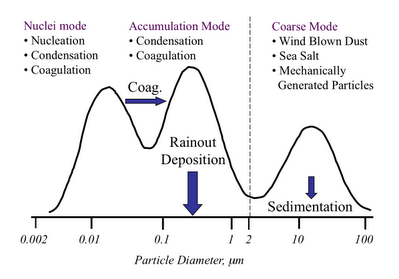
This difference in particle type based on its formation mechanism is illustrated in the figure below. This sort-of graph is a simple representation of a particle size distribution. The bottom axis is still particle diameter in micrometers. The vertical height of the curve is the number of particles present at any particular size. The higher the curve, the large the number concentration of that sized particle in any give volume of air.
The first thing to note is that there is that strange dotted line between what I am calling the accumulation mode and the coarse mode. This value of the particle diameter, generally considered to be 2.5 micrometers is what divides what I listed as smog and dust above. The reason for this divide is pretty interesting (or at least I think so.)
The particles below 2.5 microns are formed by gases that react in the atmosphere to form particles. Basically a gas molecule reacts to form a molecule that doesn’t like being in the gas phase so much, and so it condenses to form a particle. (You can think of this like steam that forms in the shower or on the mirror in the bathroom when the air can no longer hold all of the water as a gas.) These particles continue to grow by running into each other to form larger particles (coagulation) or by continued addition of the condensible gas reaction product (condensation.) The particles do not grow larger than around 2.5 microns because there is just not enough mass of the condensible gas products for them to grow larger than that. So they “accumulate” below that size – and hence termed “Accumulation Mode.”
As I mentioned below, the particles with sizes above 2.5 microns are formed by mechanical actions, breaking down larger particles into smaller ones. This is how dust is formed from sand. Sea salt particles are formed by the breaking wave action on the ocean surface. Break dust formed by wear on break pads is one of the important coarse mode particles formed by traffic. Particles that result from the subway cars rolling on their tracks is one of the primary reasons why the underground becomes so black even when the trains are electrically powered. There is a general limit on how small these types of particles can be. It takes too much energy to get the particles to smaller sizes – this mechanical abrasion process only works so far.
So to sum up – smog grows up from below and dust forms down from above – if that makes sense.
A word about this magic particle size that divides the two modes. Because this value, 2.5 microns, separates particles that are formed by two different processes, there are two separate air quality regulations that govern the two modes. The term PM2.5 refers to the total mass of particles below 2.5 microns in diameter and PM10 the total mass of particles below 10 microns. Yes, they overlap. However, particles in the coarse mode have much more mass than in the accumulation mode. (Mass scales with the cube of the diameter.) Thus having two different regulations does reflect the mass of particles formed by the two processes.